| Phase Separation |
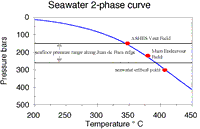
Phase SeparationThe process of phase separation, which takes place either as normal boiling or as brine condensation at supercritical pressures (see figure below), plays a crucial role in determining vent fluid composition. Elements are fractionated between the liquid and vapor phases, with ionic species partitioned into the liquid phase and volatile species into the vapor phase. Phase separation may take place under a variety of circumstances. In an aqueous salt solution, normal boiling (i.e. production of low salinity vapor) occurs when the fluid crosses the two-phase curve below critical pressure and temperature. However, if the fluid crosses the two-phase curve at a pressure and temperature above the critical point, a high-salinity brine condenses out of the fluid. These two types of phase separation produce very different chemistries.
|